Rice research could lead to better protective shields for electronics, biomedical advances
Boron nitride nanotubes sure do like to stick together. If they weren’t so useful, they could stay stuck and nobody would care.
But because they are useful, Rice University chemists have determined that surfactants — the basic compounds in soap — offer the best and easiest way to keep boron nitride nanotubes (BNNTs) from clumping. That could lead to expanded use in protective shields, as thermal and mechanical reinforcement for composite materials and in biomedical applications like delivering drugs to cells.

Rice University graduate student Ashleigh Smith McWilliams holds a vial of boron nitride nanotubes in solution. McWilliams led a Rice effort to find the best way to separate the naturally clumping nanotubes to make them more useful for manufacturing. The nanotubes turn the clear liquid surfactant white when they are dispersed.
The research led by Rice chemist Angel Martí appears this month in the Royal Society of Chemistry journal Nanoscale Advances.
BNNTs are like their better-known cousins, carbon nanotubes, because both are hydrophobic – that is, they avoid water if at all possible. So in a solution, the nanotubes will seek each other out and stick together to minimize their exposure to water.
But unlike carbon nanotubes, which can be either metallic conductors or semiconducting, BNNTs are pure insulators: Current shall not pass.
“They have super cool properties,” said lead author Ashleigh Smith McWilliams, a Rice graduate student. “They’re thermally and chemically stable and they’re a great fit for a bunch of different applications, but they’re inert and difficult to disperse in any solvent or solution.
“That makes it really difficult to make macroscopic materials out of them, which is what we would eventually like to do,” she said.
Surfactants are amphiphilic molecules, with parts that are attracted to water and parts repelled by it. BNNTs are hydrophobic, so they attract the similar part of the surfactant molecule, which wraps around the nanotube. The surfactant’s other half is hydrophilic and keeps the wrapped nanotubes separated and dispersed in solution.
Of the range of surfactants they tried, cetyl trimethyl ammonium bromide (CTAB) was best at separating BNNTs from each other completely, while Pluronic F108 put the most nanotubes – about 10 percent of the bulk – into solution.
Once separated, they can be turned into films or fibers through processes like those developed by co-author Matteo Pasquali and his Rice lab, or mixed into composites to add strength without increasing conductivity, McWilliams said. The surfactant itself can be washed or burned off when no longer needed, she said.
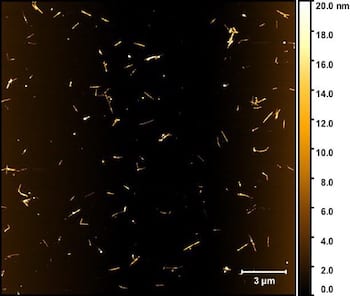
A dispersion of boron nitride nanotubes as seen through a microscope shows individual tubes and small bundles separated and solubilized by a surfactant. The process developed at Rice University will help make boron nitride nanotubes more available for applications that require nanoscale insulators. Courtesy of the Martí Research Group
A side benefit is that cationic surfactants like CTAB are particularly good at eliminating impurities like flakes of hexagonal boron-nitride (aka white graphene) from BNNTs. “That was a benefit we didn’t expect to see, but it will be useful for future applications,” McWilliams said.
“Boron nitride nanotubes are a great building block, but when you buy them, they come all clumped together,” Martí said. “You have to separate them before you can make something usable. This is what Ashleigh has achieved.”
He envisions not only ultrathin coaxial cables with carbon nanotube fibers like those from Pasquali’s lab surrounded by BNNT shells, but also capacitors of sandwiched carbon and BNNT films.
“We’ve had metallic and semiconducting carbon nanotubes for a long time, but insulating BNNTs have been like the missing link,” Martí said. “Now we can combine them to make some interesting electronics. It’s remarkable that a common surfactant found in everyday products like detergents and shampoo can also be used for advanced nanotechnology.”
Co-authors of the paper are Rice graduate student Carlos de los Reyes and undergraduate student Selin Ergülen; graduate student Lucy Liberman and Yeshayahu Talmon, professor emeritus of chemical engineering, at Technion – Israel Institute of Technology; and Pasquali, a Rice professor of chemical and biomolecular engineering, of materials science and nanoengineering and of chemistry. Martí is an associate professor of chemistry, of bioengineering and of materials science and nanoengineering.
The National Science Foundation, the Air Force Office of Scientific Research, the U.S.-Israel Binational Science Foundation and the Welch Foundation supported the research.

