NEWS RELEASE
Editor’s note: Links to a video and high-resolution images for download appear at the end of this release.
David Ruth
713-348-6327
david@rice.edu
Mike Williams
713-348-6728
mikewilliams@rice.edu
Mighty morphing materials take complex shapes
Rice University demonstrates sophisticated shape-shifters for soft robots, biomedical applications
HOUSTON – (Dec. 20, 2018) – Rice University scientists have created a rubbery, shape-shifting material that morphs from one sophisticated form to another on demand.
The shapes programmed into a polymer by materials scientist Rafael Verduzco and graduate student Morgan Barnes appear in ambient conditions and melt away when heat is applied. The process also works in reverse.
The smooth operation belies a battle at the nanoscale, where liquid crystals and the elastomer in which they’re embedded fight for control. When cool, the shape programmed into the liquid crystals dominates, but when heated, the crystals relax within the rubber band-like elastomer, like ice melting into water.
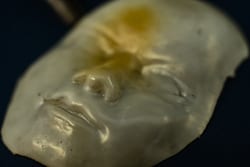
In most of the samples Barnes has made so far – including a face, a Rice logo, a Lego block and a rose – the material takes on its complex shape at room temperature, but when heated to a transition temperature of about 80 degrees Celsius (176 degrees Fahrenheit), it collapses into a flat sheet. When the heat is removed, the shapes pop back up within a couple of minutes.
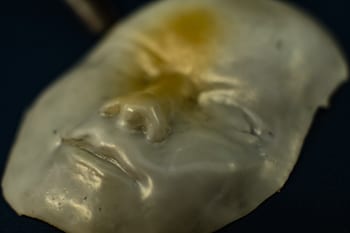
A face made of a unique polymer at Rice University takes shape when cooled and flattens when heated. The material may be useful in the creation of soft robots and for biomedical applications. Photo by Jeff Fitlow
As fanciful as this seems, the material shows promise for soft robots that mimic organisms and in biomedical applications that require materials that take pre-programmed shapes at body temperature.
The research is described in the Royal Society of Chemistry journal Soft Matter.
“These are made with two-step chemistry that has been done for a long time,” said Verduzco, a professor of chemical and biomolecular engineering and of materials science and nanoengineering. “People have focused on patterning liquid crystals, but they hadn’t thought about how these two networks interact with each other.
“We thought if we could optimize the balance between the networks – make them not too stiff and not too soft – we could get these sophisticated shape changes.”
The liquid crystal state is easiest to program, he said. Once the material is given shape in a mold, five minutes of curing under ultraviolet light sets the crystalline order. Barnes also made samples that switch between two shapes.

Rice University materials scientist Rafael Verduzco and graduate student Morgan Barnes check a sample while working on shape-shifting polymers. They have created a liquid crystal elastomer that can be molded into shapes that shift from one to another when heated. Photo by Jeff Fitlow
“Instead of simple uniaxial shape changes, where you have something that lengthens and contracts, we’re able to have something that goes from a 2D shape to a 3D shape, or from one 3D shape to another 3D shape,” she said.
The lab’s next target is to lower the transition temperature. “Activation at body temperature opens us up to a lot more applications,” Barnes said. She said tactile smartphone buttons that appear when touched or reactive braille text for the visually impaired are within reach.
She’d also like to develop a variant that reacts to light rather than heat. “We want to make it photo-responsive,” Barnes said. “Instead of heating the entire sample, you can activate only the part of the liquid crystal elastomer you want to control. That would be a much easier way to control a soft robot.”
The Welch Foundation for Chemical Research, the U.S. Army Research Office Chemical Sciences Division and the Shared Equipment Authority at Rice supported the research.
-30-
Read the abstract at https://pubs.rsc.org/en/content/articlelanding/2018/sm/c8sm02174k#!divAbstract.
Follow Rice News and Media Relations via Twitter @RiceUNews.
Video:
Video produced by Brandon Martin/Rice University
Images for download:
https://news2.rice.edu/files/2018/12/0107_SHAPES-1-web-23xjpv6.jpg
Rice University materials scientist Rafael Verduzco and graduate student Morgan Barnes check a sample while working on shape-shifting polymers. They have created a liquid crystal elastomer that can be molded into shapes that shift from one to another when heated. (Credit: Jeff Fitlow/Rice University)
https://news2.rice.edu/files/2018/12/0107_SHAPES-2-web-1sei2k2.jpg
A face made of a unique polymer at Rice University takes shape when cooled and flattens when heated. The material may be useful in the creation of soft robots and for biomedical applications. (Credit: Jeff Fitlow/Rice University)
https://news2.rice.edu/files/2018/12/0107_SHAPES-3-web-1woz8s2.jpg
Rice University graduate student Morgan Barnes monitors a sample of a shape-shifting polymer that can be molded into a shape that appears when cooled and flattens when heated. (Credit: Jeff Fitlow/Rice University)
Related materials:
Morphing material has mighty potential: http://news.rice.edu/2013/12/06/morphing-material-has-mighty-potential-2/
Verduzco Laboratory: http://verduzcolab.blogs.rice.edu
Rice Department of Chemical and Biomolecular Engineering: https://chbe.rice.edu
George R. Brown School of Engineering: https://engineering.rice.edu
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,962 undergraduates and 3,027 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 2 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance. To read “What they’re saying about Rice,” go to http://tinyurl.com/RiceUniversityoverview.