Rice scientists’ approach to cell dynamics builds new view of lifetime risk
When do cancer-prone cells turn into full-blown cancer? A Rice University scientist and his colleague believe there’s a way to know.
It may become possible for biomarkers in blood to reveal whether mutated cells have turned a corner toward forming tumors, and how long the process — depending on the type of cancer — is likely to take. That could give patients a sense of the risk they face before they become ill.
Rice chemist Anatoly Kolomeisky, with postdoctoral research associate Hamid Teimouri and graduate alumna Maria Kochugaeva, directed their theoretical expertise in modeling random (stochastic) processes to the problem of why cancerous cells that are usually destroyed by the body’s immune system sometimes overcome a gauntlet of defenses to become tumors.
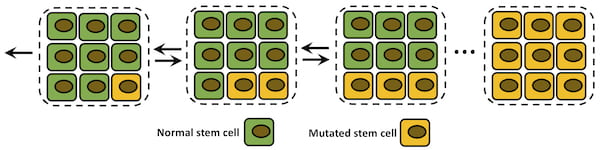
A schematic shows a single mutation fixation process in a tissue compartment. Normal stem cells are green, and mutated cells are yellow. Rice University researchers used a discrete-state stochastic model to see how cancer-leaning mutations affected the likelihood of cells turning tissue into a tumor, and how well the model correlates with widely used calculations of cancer lifetime risks. Illustration by Hamid Teimouri
The study in Scientific Reports is intended to clarify microscopic aspects of cancer initiation, the point at which random mutations become “fixed” in cells, which pass them on and eventually overwhelm tissue.

Hamid Teimouri
As part of their study, the researchers calculated fixation probabilities for 28 types of cancer to see how they correlate with available clinical data on cancer lifetime risks — and found that correlation was anything but sure.
“The problem with cancer is that we’re detecting it too late,” said Kolomeisky, a professor of chemistry and chairman of the department in Rice’s Wiess School of Natural Sciences. The researchers argue their “convenient, simple and versatile” method to evaluate cancer initiation dynamics should incorporate initiation dynamics for specific cancer types as well as established lifetime risks.
Kolomeisky has a long track record of discovery related to cellular mechanisms, especially related to motor proteins that transport cargo in cells and genome editing. Inspired by an off-hand comment from a colleague that his calculations looked like they could relate to cancer, Kolomeisky set off to find out if his mathematical approach could be applied to cancer initiation dynamics.
Based on their formulas, the researchers developed a chart correlating lifetime risks and initiation times for 28 types of cancer — and found very little to connect the two. “It indicates that cancer lifetime risks alone cannot be utilized to evaluate the danger of getting cancer,” the researchers wrote, suggesting fixation times that indicate tumor initiation are a more important metric.

Maria Kochugaeva
For example, the chart shows that colorectal adenocarcinoma with FAP carries the highest lifetime risk, but its fixation time of 15 years is nearly three times that of duodenum adenocarcinoma with FAP, which is thought to have a much lower lifetime risk. At the other end of the fixation scale are hepatocellular carcinomas, at more than 31,000 years.
Their calculations compared the “fitness” of individual cells with cancer-related mutations to normal cells without, and how fast they divided. Tissue with mutant cells that divided at a 1-to-1 ratio with normal cells were the slowest to “fix” tumors.
The researchers assumed mutant cells with a speed advantage would fix tumors faster, and they were right. But they were surprised when their models showed cells with disadvantageous mutations (slower-than-normal division) were also occasionally faster than normal cells to fix.
“People assume, usually subconsciously, that something more probable will happen faster,” Kolomeisky said. “But we found that is not the case for cancers. Doctors shouldn’t make decisions simply on lifetime risk, as is typically done, but also on the dynamics we calculated as our main results.

Anatoly Kolomeisky
“The main points are that we can calculate, with a lot of assumptions, the average time before cancer will start,” he said. “We also argue that the probability of cancer does not typically correlate well with the time it starts. Something more probable is not necessarily fast.”
Mutant cells that release biomarkers to the bloodstream may provide a better way to spot precancerous conditions.
“In the future, this could become part of personalized medicine,” Kolomeisky said. “A doctor gives you a test and checks for the fraction of mutated cells in your tissues. Then you can estimate how long it would take before the mutated cells will occupy the whole tissue. If it’s 100 years, you probably should not worry too much. But if it’s one year, then you might have to take drastic measures.”
He said the model will certainly improve as more relevant parameters are incorporated. But anything it produces now and in the future should not deter one from a healthy lifestyle.
“We’re not saying that you will or won’t get cancer no matter what you do,” Kolomeisky said. “Just that the probability is different. If you smoke, if you don’t eat well or don’t exercise, the probability will be higher.”
Teimouri earned his doctorate in the Kolomeisky lab. Kochugaeva is now a postdoctoral associate at Yale University’s Systems Biology Institute.
The Welch Foundation, the National Science Foundation and Rice’s Center for Theoretical Biological Physics supported the research.

