Rice, UTHealth discovery could aid fight against multidrug-resistant VRE
Researchers from Rice University and the University of Texas Health Science Center at Houston spent more than eight years trying to solve a puzzle: How could a deadly superbug known as VRE become resistant to a new antibiotic in less than five years? The answer surprised everyone.
In a study online this week in the Proceedings of the National Academy of Sciences, Rice biochemist Yousif Shamoo, UTHealth infectious disease expert Dr. Cesar Arias and colleagues report that VRE, or vancomycin-resistant Enterococci, employs a unique strategy not previously reported in an infectious bacterium: It deploys legions of sentinel proteins that stand watch outside the cell and give advanced warning of antibiotic attacks. The research is available online in the Proceedings of the National Academy of Sciences.
“We spent years looking inside the cell for this thing,” Shamoo said of the sentinel protein LiaX. Shamoo’s team and their collaborators discovered LiaX in 2012 and began working together to find out how VRE, a multidrug-resistant superbug classified as a serious threat by the U.S. Centers for Disease Control (CDC), developed resistance to daptomycin within a few years of its debut in 2003.
“We all have Enterococci in our gut right now,” Shamoo said. “And as long as it stays in our gut, it’s all good. But if they get into your bloodstream, and your immune system is compromised and fails to get rid of them, Enterococci can cause heart valve infections and bacteremia, which can kill you in a matter of days.”
Antibiotic drugs, which went into wide use after World War II, attack bacteria directly and aid our immune systems in clearing infections. But bacteria can evolve to defend themselves from antibiotics. The CDC estimates that antibiotic-resistant pathogens sicken more than 2.8 million people and kill almost 36,000 in the U.S. each year. VRE accounted for about 5,400 U.S. deaths in 2017. The organism’s moniker stems from the widespread resistance it developed to vancomycin late last century. Prior to the appearance of daptomycin, vancomycin was often used as a “drug of last resort” when all other treatment options failed.
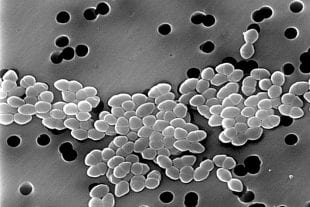
Vancomycin-resistant Enterococci, as imaged with a scanning electron microscope. (Photo by Janice Haney Carr/CDC)
“Daptomycin is an antibiotic that was originally isolated out of a soil bacterium called streptomyces,” Shamoo said. “Evolutionarily, Enterococci should never have encountered daptomycin. So one of the early mysteries was how it became daptomycin-resistant so quickly.”
When his and Arias’ labs identified LiaX in 2012, it didn’t have a name. Shamoo’s lab often conducts “directed evolution” experiments in which organisms are forced to adapt to a threat. By repeatedly exposing non-daptomycin-resistant VRE strains to the drug and observing how they evolved, Shamoo’s team created a list of genes that often changed in the resistant strains. LiaX popped up on the list and was interesting, in part, because it was an unknown.
“We didn’t know how important it was at the time,” he said. “It’s part of a group of genes that we all thought were involved in resistance. Some of the others we kind of understood, and this one was unusual. It drew our curiosity because it conferred resistance in a way that we didn’t understand.”
Arias, Shamoo and their students continued their research, and published more than a dozen studies about VRE and daptomycin resistance.
“Cesar and I have been working a long time on this,” Shamoo said. “All of our papers are about this organism and trying to understand not just how it becomes resistant to antibiotics but what we can do about it. Some of the work is very basic research, but it’s always directed towards this very clinical outcome. Based on some of our previous work, for instance, we made recommendations about ways to combat resistance by using daptomycin differently.”

The U.S. Centers for Disease Control summarized key facts about vancomycin-resistant Enterococci (VRE) on page 93 of its report “Antibiotic Resistance Threats in the United States, 2019” (Courtesy of the CDC)
PNAS study co-author Milya Davlieva, a former senior scientist in Shamoo’s lab, “did a lot of this very early work, making the protein and understanding its biochemistry,” Shamoo said. Study lead author Ayesha Khan, a graduate student in Arias’ lab, had a key insight for the new study.
“She noticed that you didn’t need the cells to get the response,” Shamoo said. “You could grow them in a flask, get rid of all the cells, take the spent media and elicit the response in new cells.
“There was something in the media that had the ability to turn on the defenses in a new bunch of cells,” he said. It turned out to be LiaX, the protein that the two groups had searched for inside the cell for so many years.
“We call it a sentinel protein,” Shamoo said. “It goes out, and it scouts. When it finds a threat, it signals the cell to turn on its defenses.”
From studies, the researchers know the “alarm” signal causes VRE to activate genes that modify its cell wall, or membrane, to defend itself from daptomycin. Shamoo said bacteria often make proteins that sense attacks and activate defenses, but these typically dot the surface of the cell and have direct links with the interior of the cell. LiaX is the first example of a sentinel protein that’s sent out into to the environment to stand watch.
“It’s unusual,” he said. “That’s why it took us so long to figure it out.”
Another key finding — the one that answers the longstanding question about how quickly VRE adapted to daptomycin — is that LiaX guards against more than daptomycin.
“It also detects a peptide called LL-37,” Shamoo said. “This is something that’s made by human macrophages, which are part of our immune system. Macrophages make LL-37 and secrete it into their environment to kill bacteria, and it turns out that LiaX also detects that.”
Shamoo said because Enterococci live in us on a daily basis, their defense systems have evolved and been shaped by threats from our immune systems, like LL-37. “They have this really great system that allows them to recognize these attacks, and LiaX is at the crux of that,” he said.
That discovery opens the door to many follow-up studies. Open questions include how LiaX is activated, and whether that differs if it’s activated by LL-37 or daptomycin. And there are also questions about how the finding might lead to better treatments for people infected with VRE.
“The importance of this paper goes beyond this one drug,” Shamoo said. “It’s actually about how this organism responds to a whole class of attacks. LiaX is part of how these organisms defeat both the drug and our immune system.”
Study co-authors include Orville Pembertone of Rice; Diana Panesso, William Miller, Melissa Cruz, April Nguyen, Sara Siegel, Kavindra Singh, Danielle Garsin and Truc Tran, all of UTHealth; Sandra Rincon, Lorena Diaz, Jinnethe Reyes and Rafael Rios, all of El Bosque University in Bogota, Columbia; Paul Planet of the University of Pennsylvania; Apurva Narechania of the American Museum of Natural History in New York; Mauricio Latorre of the University of Chile; and Hung Ton-That of UCLA.
The research was supported by the National Institutes of Health and the University of Texas System.


