Jeff Falk
713-348-6775
jfalk@rice.edu
Jade Boyd
713-348-6778
jadeboyd@rice.edu
Rice researchers demo solar water-splitting technology
Process uses light-harvesting nanoparticles, captures energy from ‘hot electrons’
HOUSTON — (Sept. 4, 2015) — Rice University researchers have demonstrated an efficient new way to capture the energy from sunlight and convert it into clean, renewable energy by splitting water molecules.
The technology, which is described online in the American Chemical Society journal Nano Letters, relies on a configuration of light-activated gold nanoparticles that harvest sunlight and transfer solar energy to highly excited electrons, which scientists sometimes refer to as “hot electrons.”
“Hot electrons have the potential to drive very useful chemical reactions, but they decay very rapidly, and people have struggled to harness their energy,” said lead researcher Isabell Thomann, assistant professor of electrical and computer engineering and of chemistry and materials science and nanoengineering at Rice. “For example, most of the energy losses in today’s best photovoltaic solar panels are the result of hot electrons that cool within a few trillionths of a second and release their energy as wasted heat.”
Capturing these high-energy electrons before they cool could allow solar-energy providers to significantly increase their solar-to-electric power-conversion efficiencies and meet a national goal of reducing the cost of solar electricity.
In the light-activated nanoparticles studied by Thomann and colleagues at Rice’s Laboratory for Nanophotonics (LANP), light is captured and converted into plasmons, waves of electrons that flow like a fluid across the metal surface of the nanoparticles. Plasmons are high-energy states that are short-lived, but researchers at Rice and elsewhere have found ways to capture plasmonic energy and convert it into useful heat or light. Plasmonic nanoparticles also offer one of the most promising means of harnessing the power of hot electrons, and LANP researchers have made progress toward that goal in several recent studies.
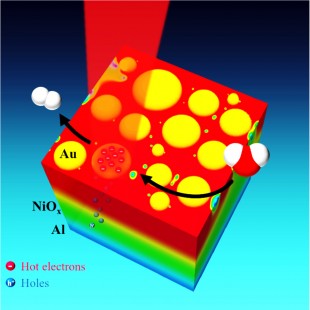
Rice University researchers have demonstrated an efficient new way to capture the energy from sunlight and convert it into clean, renewable energy by splitting water molecules.
Thomann and her team, graduate students Hossein Robatjazi, Shah Mohammad Bahauddin and Chloe Doiron, created a system that uses the energy from hot electrons to split molecules of water into oxygen and hydrogen. That’s important because oxygen and hydrogen are the feedstocks for fuel cells, electrochemical devices that produce electricity cleanly and efficiently.
To use the hot electrons, Thomann’s team first had to find a way to separate them from their corresponding “electron holes,” the low-energy states that the hot electrons vacated when they received their plasmonic jolt of energy. One reason hot electrons are so short-lived is that they have a strong tendency to release their newfound energy and revert to their low-energy state. The only way to avoid this is to engineer a system where the hot electrons and electron holes are rapidly separated from one another. The standard way for electrical engineers to do this is to drive the hot electrons over an energy barrier that acts like a one-way valve. Thomann said this approach has inherent inefficiencies, but it is attractive to engineers because it uses well-understood technology called Schottky barriers, a tried-and-true component of electrical engineering.
“Because of the inherent inefficiencies, we wanted to find a new approach to the problem,” Thomann said. “We took an unconventional approach: Rather than driving off the hot electrons, we designed a system to carry away the electron holes. In effect, our setup acts like a sieve or a membrane. The holes can pass through, but the hot electrons cannot, so they are left available on the surface of the plasmonic nanoparticles.”

Rice University researchers (clockwise from left) Chloe Doiron, Hossein Robatjazi, Shah Mohammad Bahauddin and Isabell Thomann.
The setup features three layers of materials. The bottom layer is a thin sheet of shiny aluminum. This layer is covered with a thin coating of transparent nickel-oxide, and scattered atop this is a collection of plasmonic gold nanoparticles — puck-shaped disks about 10 to 30 nanometers in diameter.
When sunlight hits the discs, either directly or as a reflection from the aluminum, the discs convert the light energy into hot electrons. The aluminum attracts the resulting electron holes and the nickel oxide allows these to pass while also acting as an impervious barrier to the hot electrons, which stay on gold. By laying the sheet of material flat and covering it with water, the researchers allowed the gold nanoparticles to act as catalysts for water splitting. In the current round of experiments, the researchers measured the photocurrent available for water splitting rather than directly measuring the evolved hydrogen and oxygen gases produced by splitting, but Thomann said the results warrant further study.
“Utilizing hot electron solar water-splitting technologies we measured photocurrent efficiencies that were on par with considerably more complicated structures that also use more expensive components,” Thomann said. “We are confident that we can optimize our system to significantly improve upon the results we have already seen.”
Robatjazi is a graduate student in electrical and computer engineering, Bahauddin is a graduate student in physics and astronomy and Doiron is a graduate student in applied physics. The research was supported by the Welch Foundation and a National Science Foundation CAREER Award.
-30-
High-resolution IMAGES are available for download at:
https://news2.rice.edu/files/2015/08/0901-ELECTRO-team-lg.jpg
CAPTION: Rice University researchers (clockwise from left) Chloe Doiron, Hossein Robatjazi, Shah Mohammad Bahauddin and Isabell Thomann.
CREDIT: Jeff Fitlow/Rice University
https://news2.rice.edu/files/2015/08/0901-ELECTRO-color-lg.jpg
CAPTION: Rice University researchers have demonstrated an efficient new way to capture the energy from sunlight and convert it into clean, renewable energy by splitting water molecules.
CREDIT: I. Thomann/Rice University
https://news2.rice.edu/files/2014/06/0616_THOMANN-Isabell3-lg.jpg
CAPTION: Isabell Thomann
CREDIT: Jeff Fitlow/Rice University
A copy of the NanoLetters paper, “Direct Plasmon-Driven Photoelectrocatalysis,” is available at:
http://pubs.acs.org/doi/abs/10.1021/acs.nanolett.5b02453
This release can be found online at news-network.rice.edu/news.
Follow Rice News and Media Relations via Twitter @RiceUNews
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,888 undergraduates and 2,610 graduate students, Rice’s undergraduate student-to-faculty ratio is 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for best quality of life and for lots of race/class interaction by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance. To read “What they’re saying about Rice,” go here.


